Zöld Izrael » Innováció, Kutatás, Természetes erőforrások, Zöld energia » Storing Solar Energy in Rust, From Israel
Storing Solar Energy in Rust, From Israel
Scientists at Technion, Israel’s institute of technology recently found a new way to store solar energy. Their method utilizes a substance that some of us are all too familiar with, iron oxide– otherwise known as rust. This research entitled Resonant light trapping in ultrathin films for water was published in the November 11, 2012 issue of Nature Materials and may help solve the problem of solar energy storage by enabling a more efficient and direct conversion between solar energy and hydrogen.
I grew up in a region that was once known as the rust belt. Iron foundries, heavy industry and heavy cars were plentiful in the upper Midwestern US. Winters were icy so governments used salt to help make the roads safer. Unfortunately this also made automobiles rustier. Comedian Dave Barry once joked that American cars were made out of compressed rust. Salt-encrusted lumps of grey slush clung to the bottoms of cars and performed the alchemy of converting iron and gleaming steel into crumbling heaps of orange-red rust.
Actually it wasn’t alchemy, it was ordinary chemistry which is a little too complicated to explain here, but parts of the reaction can be simplified to something like this:
2Fe(s) + 2H2O(l) + O2(g) → 2Fe2+(aq) + 4OH-(aq) {a bit more magic} → Fe2O3 .nH2O
Iron, water and oxygen combine to make a solution which dries to become rust.
All about rust
Rust crumbles in your hand and stains your skin, your clothes and concrete structures. Grocery stores once sold a chemical which was supposed to remove rust stains but it was also powerful enough to eat through metal, skin and glass. A form of rust was used in audio cassette tapes, 8-tracks, floppy disks and the hard drive which is storing this article. In 1976 NASA’s Viking lander arrived on Mars and found– rust.
The 1976 Buick I drove to prom had lost the bottom half of its passenger doors to rust. I once connected a voltmeter to some bolts straddling the rusted-out floor of my father’s car and found that the electrochemical process which gradually turned his 1969 AMC Rambler into a heap of rust also generated about 1/2 volt of electricity. I never patented this corrosion-powered “battery car” as a form of planned-obsolescence Detroit would have loved but I’m beginning to wish I’d saved some of that rust. Will it soon become as valuable as platinum was during the cold fusion fiasco? Might the rust belt prosper from this abundant resource just as Saudi Arabia did from oil and the Canadian Yukon did from gold?
Maybe not. Photovoltaic cells are made out of silicon which is found in desert sand but the price of sand hasn’t gone up very much even as the photovoltaic market grows. It turns out that just as it is for photovoltaic silicon, carbon nanotubes, diamonds and coal; the secret is not in the ingredients it is in the preparation. The Technion researchers discovered how to turn something ordinary into something useful by applying materials science.
The paper’s abstract offers a clue:
Semiconductor photoelectrodes for solar hydrogen production by water photoelectrolysis must employ stable, non-toxic, abundant and inexpensive visible-light absorbers. Iron oxide (α-Fe2O3) is one of few materials meeting these requirements, but its poor transport properties present challenges for efficient charge-carrier generation, separation, collection and injection. Here we show that these challenges can be addressed by means of resonant light trapping in ultrathin films designed as optical cavities.
Interference between forward- and backward-propagating waves enhances the light absorption in quarter-wave or, in some cases, deeper subwavelength films, amplifying the intensity close to the surface wherein photogenerated minority charge carriers (holes) can reach the surface and oxidize water before recombination takes place.
The researchers are making use of rust’s light absorbing properties which make those stains so visible on a T-shirt. They’re making use of its stability. Water and oxygen can turn a car into rust, but even a large dose of rust remover won’t turn that rust back into a car. They’re also making use of thin-film optical interference. Look at a pair of anti-reflection eyeglasses, the lens of a camera or binoculars, a soap bubble or a thin oil slick floating on a puddle and you’ll see one of the effects these scientists were taking advantage of.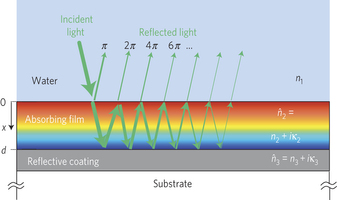
When light comes across a thin semi-transparent film, some of the light is reflected off the bottom of the film, some is reflected off the top. If the film is just the right thickness, light of a certain wavelength can be made to constructively interfere with its reflection, making that color brighter or destructively interfere making that color dimmer.
In this case the scientists have found a way to enhance the light intensity exactly where it is needed to help separate the water’s hydrogen from its oxygen. The hydrogen can then be stored and used to generate energy when the sun isn’t shining. It’s a pretty neat trick for a lump of rust. Kudos to Hen Dotan, Ofer Kfir, Elad Sharlin, Oshri Blank, Moran Gross, Irina Dumchin, Guy Ankonina and Avner Rothschild for their research.
Forrás: http://www.greenprophet.com/2012/11/energy-solar-rust-israel/
Kategória: Innováció, Kutatás, Természetes erőforrások, Zöld energia








